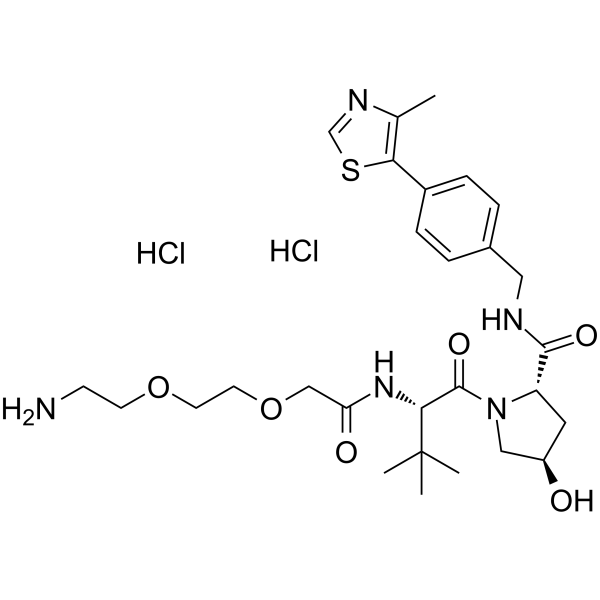
(S,R,S)-AHPC-PEG2-NH2 dihydrochloride
CAS No. 2341796-76-5
(S,R,S)-AHPC-PEG2-NH2 dihydrochloride ( VH032-PEG2-NH2 dihydrochloride | VH032-linker 12 | VH032 amide-PEG2-amine )
产品货号. M27014 CAS No. 2341796-76-5
(S,R,S)-AHPC-PEG2-NH2 二盐酸盐是一种合成的 E3 连接酶配体-连接子缀合物,其中包含基于 (S,R,S)-AHPC 的 VHL 配体和 2 单元 PEG 连接子。
纯度: >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| 规格 | 价格/人民币 | 库存 | 数量 |
| 5MG | ¥275 | 有现货 |


|
| 10MG | ¥446 | 有现货 |


|
| 25MG | ¥915 | 有现货 |


|
| 50MG | ¥1442 | 有现货 |


|
| 100MG | ¥2066 | 有现货 |


|
| 200MG | 获取报价 | 有现货 |


|
| 500MG | 获取报价 | 有现货 |


|
| 1G | 获取报价 | 有现货 |


|
生物学信息
-
产品名称(S,R,S)-AHPC-PEG2-NH2 dihydrochloride
-
注意事项本公司产品仅用于科研实验,不得用于人体或动物的临床与诊断
-
产品简述(S,R,S)-AHPC-PEG2-NH2 二盐酸盐是一种合成的 E3 连接酶配体-连接子缀合物,其中包含基于 (S,R,S)-AHPC 的 VHL 配体和 2 单元 PEG 连接子。
-
产品描述(S,R,S)-AHPC-PEG2-NH2 dihydrochloride is a synthesized E3 ligase ligand-linker conjugate incorporating the (S,R,S)-AHPC based VHL ligand and 2-unit PEG linker.(In Vitro):PROTACs exploit the intracellular ubiquitin-proteasome system to selectively degrade target proteins.
-
体外实验PROTACs contain two different ligands connected by a linker; one is a ligand for an E3 ubiquitin ligase and the other is for the target protein. PROTACs exploit the intracellular ubiquitin-proteasome system to selectively degrade target proteins.
-
体内实验——
-
同义词VH032-PEG2-NH2 dihydrochloride | VH032-linker 12 | VH032 amide-PEG2-amine
-
通路Others
-
靶点Other Targets
-
受体——
-
研究领域——
-
适应症——
化学信息
-
CAS Number2341796-76-5
-
分子量648.64
-
分子式C28H43Cl2N5O6S
-
纯度>98% (HPLC)
-
溶解度——
-
SMILESO=C([C@H]1N(C([C@@H](NC(COCCOCCN)=O)C(C)(C)C)=O)C[C@H](O)C1)NCC2=CC=C(C3=C(C)N=CS3)C=C2.Cl.Cl
-
化学全称——
运输与储存
-
储存条件(-20℃)
-
运输条件With Ice Pack
-
稳定性≥ 2 years
参考文献
1.Sagong HY, Kim KJ. Structural Insights into Substrate Specificity of Cystathionine γ-Synthase from Corynebacterium glutamicum. J Agric Food Chem. 2017 Jul 26;65(29):6002-6008.
产品手册




关联产品
-
Dioscoreside C
Dioscoreside C
-
[Tyr27]-a-CGRP (27-3...
(Tyr27)-α-CGRP (27-37) (canine, mouse, rat) 是一种多肽,能够由多肽筛选发现。多肽筛选是一种主要通过免疫测定法而集合活性多肽的研究工具。多肽筛选可用于蛋白互作、功能分析,抗原表位筛选,特别是活性分子研究和开发领域。
-
Dcimc chloride
3-(2, 6-二氯苯基)-5-甲基异恶唑-4-碳酰氯具有抗生素活性。



 021-51111890
021-51111890 购物车()
购物车()
 sales@molnova.cn
sales@molnova.cn







